NAFDAC Releases List of 101 Banned and Suspended Products in Nigeria
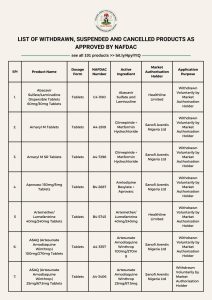
The National Agency for Food and Drug Administration and Control (NAFDAC) has made public a comprehensive list of products whose registrations have either been withdrawn, suspended, or cancelled across Nigeria.
PulseNets learnt from the agency’s official website on Tuesday that the affected medical products are no longer permitted for manufacture, importation, exportation, advertisement, distribution, sale, or use within the country.
Among the highlighted products obtained by PulseNets are Artemether/Lumefantrine 40mg/240mg Tablets, Amaryl M SR Tablets, Abacavir Sulfate/Lamivudine Dispersible Tablets 60mg/30mg, Aprovasc 150mg/5mg Tablets, ASAQ (Artesunate Amodiaquine Winthrop) 100mg/270mg Tablets, Betopic Eye Drops, Efavirenz 600mg Tablets, and Flagyl Suspension.
Other items on the list, PulseNets reported, include Iliadin Adult 0.05% Metered Nose Spray, Invanz 1g Injections, and Invega (Paliperidone) 3mg Extended Release Tablets.
Speaking on the development, NAFDAC explained to PulseNets that
“a product’s certificate of registration is considered withdrawn when the market authorisation holder voluntarily discontinues its use.”
The agency further told PulseNets that
“a certificate may also be suspended if the conditions under which it was originally granted are no longer being met, giving NAFDAC the authority to enforce such a suspension.”
Also Read: NAFDAC Bills Firms for Raiding Them — Ex-NACCIMA President Alleges
The full list, which contains 101 pharmaceutical and healthcare products affected by the withdrawal, suspension, or outright cancellation of registration, has been published on the regulator’s website for public access and compliance.